Details of the Drug Combinations
General Information of This Drug (ID: DMIZ6W2)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Baccidal; Barazan; Chibroxin; Fulgram; Lexinor; NFLX; Norflo; Norfloxacine; Norfloxacino; Norfloxacinum; Noroxin; Sebercim; Merck Brand of Norfloxacin; Norfloxacin Merck Brand; AM 0715; AM 715; AM0715; MK 0366; MK 366; MK0366; MK366; AM-0715; AM-715; Chibroxin (TN); Insensye (TN); MK-0366; MK-366; Norflohexal (TN); Norfloxacine [INN-French]; Norfloxacino [INN-Spanish]; Norfloxacinum [INN-Latin]; Norfocin (TN); Noroxin (TN); Nufloxib (TN); Roxin (TN); Utin (TN); Utinor (TN); Apo-Norflox (TN); Norfloxacin (JP15/USP/INN); Norfloxacin [USAN:BAN:INN:JAN]; Chibroxin, MK-366, Baccidal, Sebercim, Zoroxin, Norfloxacin; 1,4-Dihydro-1-ethyl-6-fluoro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid; 1-Ethyl-6-fluor-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-chinolincarbonsaeure; 1-Ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid; 1-Ethyl-6-fluoro-1,4-dihydro-4-oxo-7-[1-piperazinyl]-3-quinoline-carboxylic acid; 1-ethyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylic acid; 1-ethyl-6-fluoro-4-oxo-7-piperazin-1-yl-1,4-dihydroquinoline-3-carboxylic acid; 1-ethyl-6-fluoro-4-oxo-7-piperazin-1-ylquinoline-3-carboxylic acid
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antibiotics
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
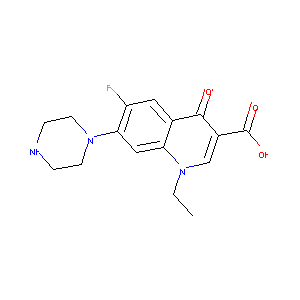 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
2 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||
References
