Details of the Drug Combinations
General Information of This Drug (ID: DMJBOCR)
| Drug Name | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Levocarnitine; L-carnitine; 541-15-1; vitamin BT; (R)-Carnitine; Carnitor; (-)-Carnitine; Carnitine; Karnitin; (-)-L-Carnitine; L-(-)-Carnitine; Levocarnitina; Carnitene; Levocarnitinum; Metina; L-Carnitine inner salt; Carniking; Carnovis; Carnitolo; Carnilean; Carrier; Lefcar; Carnitine, (-)-; L-carnitine Base; Levocarnitinum [Latin]; Levocarnitina [Spanish]; ST 198; L(-)-Carnitine; Carniking 50; (3R)-3-hydroxy-4-(trimethylammonio)butanoate; Levocarnitine [USAN:INN]; gamma-Trimethyl-beta-hydroxybutyrobetaine; bicarnesine; Nicetile (TN); L-Carnitine
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||
| Structure |
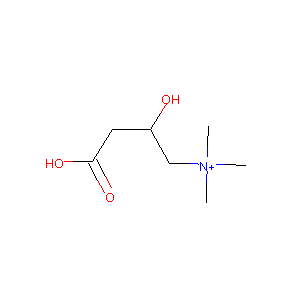 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
