Details of the Drug
General Information of Drug (ID: DMJBOCR)
| Drug Name |
Levacecarnine hci
|
||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Levocarnitine; L-carnitine; 541-15-1; vitamin BT; (R)-Carnitine; Carnitor; (-)-Carnitine; Carnitine; Karnitin; (-)-L-Carnitine; L-(-)-Carnitine; Levocarnitina; Carnitene; Levocarnitinum; Metina; L-Carnitine inner salt; Carniking; Carnovis; Carnitolo; Carnilean; Carrier; Lefcar; Carnitine, (-)-; L-carnitine Base; Levocarnitinum [Latin]; Levocarnitina [Spanish]; ST 198; L(-)-Carnitine; Carniking 50; (3R)-3-hydroxy-4-(trimethylammonio)butanoate; Levocarnitine [USAN:INN]; gamma-Trimethyl-beta-hydroxybutyrobetaine; bicarnesine; Nicetile (TN); L-Carnitine
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||
| Structure |
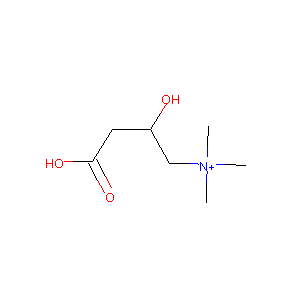 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 161.2 | |||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.2 | ||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
|---|---|---|---|---|---|
| 2 | A standard database for drug repositioning. Sci Data. 2017 Mar 14;4:170029. | ||||
| 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4780). | ||||
| 4 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | Central ghrelin regulates peripheral lipid metabolism in a growth hormone-independent fashion. Endocrinology. 2009 Oct;150(10):4562-74. | ||||
| 7 | Molecular and functional analysis of SLC25A20 mutations causing carnitine-acylcarnitine translocase deficiency. Hum Mutat. 2004 Oct;24(4):312-20. | ||||
| 8 | Histological characterization of orphan transporter MCT14 (SLC16A14) shows abundant expression in mouse CNS and kidney. BMC Neurosci. 2016 Jul 1;17(1):43. | ||||
| 9 | The human carnitine transporter SLC22A16 mediates high affinity uptake of the anticancer polyamine analogue bleomycin-A5. J Biol Chem. 2010 Feb 26;285(9):6275-84. | ||||
| 10 | A polarized localization of amino acid/carnitine transporter B(0,+) (ATB(0,+)) in the blood-brain barrier. Biochem Biophys Res Commun. 2008 Nov 14;376(2):267-70. | ||||
| 11 | Spontaneous development of intestinal and colonic atrophy and inflammation in the carnitine-deficient jvs (OCTN2(-/-)) mice. Mol Genet Metab. 2007 Dec;92(4):315-24. | ||||
| 12 | Colistin is substrate of the carnitine/organic cation transporter 2 (OCTN2, SLC22A5). Drug Metab Dispos. 2017 Dec;45(12):1240-1244. | ||||
| 13 | Modulation of ethanol-mediated CYP2E1 induction by clofibrate and L-carnitine in rat liver. Biol Pharm Bull. 1993 Dec;16(12):1240-3. | ||||
| 14 | Structural and mutational characterization of L-carnitine binding to human carnitine acetyltransferase. J Struct Biol. 2004 Jun;146(3):416-24. | ||||
| 15 | The disruption of L-carnitine metabolism by aluminum toxicity and oxidative stress promotes dyslipidemia in human astrocytic and hepatic cells. Toxicol Lett. 2011 Jun 24;203(3):219-26. doi: 10.1016/j.toxlet.2011.03.019. Epub 2011 Mar 23. | ||||
| 16 | Clozapine-induced reduction of l-carnitine reabsorption via inhibition/down-regulation of renal carnitine/organic cation transporter 2 contributes to liver lipid metabolic disorder in mice. Toxicol Appl Pharmacol. 2019 Jan 15;363:47-56. doi: 10.1016/j.taap.2018.11.007. Epub 2018 Nov 19. | ||||
| 17 | Transcellular movement of hydroxyurea is mediated by specific solute carrier transporters. Exp Hematol. 2011 Apr;39(4):446-56. | ||||
