Details of the Drug Combinations
General Information of This Drug (ID: DMJH792)
| Drug Name | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Pitavastatin; Itavastatin; Livalo; NK 104; Pitavastatin [INN]; Pitavastatin calcium; UNII-M5681Q5F9P; NK-104; C25H24FNO4; M5681Q5F9P; Zypitamag; Flovas; (3R,5S,6E)-7-(2-Cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl)-3,5-dihydroxyhept-6-enoic acid; P 872441; P-872441; (3R,5S,6E)-7-[2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl]-3,5-dihydroxyhept-6-enoic acid; ( )-(3R,5S,6E)-7-(2-Cyclopropyl-4-(4-fluorophenyl)-3-quinolyl)-3,5-dihydroxy-6-heptenoic acid; NK 104 (acid); Pitavastatin calcium (JAN)
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Structure |
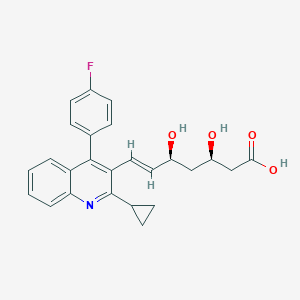 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
6 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
