| 1 |
ClinicalTrials.gov (NCT01695954) Pharmacokinetic Study of Pitavastatin and Ritonavir-Boosted Darunavir or Efavirenz
|
| 2 |
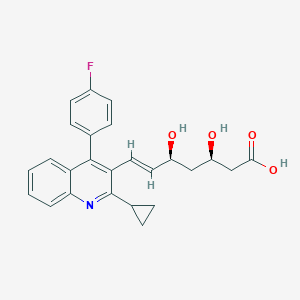
Pitavastatin FDA Label
|
| 3 |
Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77.
|
| 4 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 5 |
Involvement of BCRP (ABCG2) in the biliary excretion of pitavastatin. Mol Pharmacol. 2005 Sep;68(3):800-7.
|
| 6 |
ABCG2: a perspective. Adv Drug Deliv Rev. 2009 Jan 31;61(1):3-13.
|
| 7 |
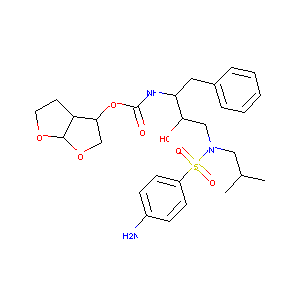
Prediction of the in vivo OATP1B1-mediated drug-drug interaction potential of an investigational drug against a range of statins. Eur J Pharm Sci. 2012 Aug 30;47(1):244-55.
|
| 8 |
Transporter-mediated influx and efflux mechanisms of pitavastatin, a new inhibitor of HMG-CoA reductase. J Pharm Pharmacol. 2005 Oct;57(10):1305-11.
|
| 9 |
Impact of OATP transporters on pharmacokinetics. Br J Pharmacol. 2009 Oct;158(3):693-705.
|
| 10 |
Intestinal absorption of HMG-CoA reductase inhibitor pitavastatin mediated by organic anion transporting polypeptide and P-glycoprotein/multidrug resistance 1. Drug Metab Pharmacokinet. 2011;26(2):171-9.
|
| 11 |
Effect of pitavastatin on transactivation of human serum paraoxonase 1 gene. Metabolism. 2005 Feb;54(2):142-50.
|
| 12 |
Early identification of clinically relevant drug interactions with the human bile salt export pump (BSEP/ABCB11). Toxicol Sci. 2013 Dec;136(2):328-43.
|
| 13 |
Regulation mechanism of ABCA1 expression by statins in hepatocytes. Eur J Pharmacol. 2011 Jul 15;662(1-3):9-14. doi: 10.1016/j.ejphar.2011.04.043. Epub 2011 May 1.
|
| 14 |
Statin-induced Mitochondrial Priming Sensitizes Multiple Myeloma Cells to BCL2 and MCL-1 Inhibitors. Cancer Res Commun. 2023 Dec 8;3(12):2497-2509. doi: 10.1158/2767-9764.CRC-23-0350.
|
| 15 |
2006 drug approvals: finding the niche. Nat Rev Drug Discov. 2007 Feb;6(2):99-101.
|
| 16 |
Impact of drug transporters on cellular resistance towards saquinavir and darunavir. J Antimicrob Chemother. 2010 Nov;65(11):2319-28.
|
| 17 |
Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev. 2011 Mar;63(1):157-81.
|
| 18 |
P-glycoprotein mediates efflux transport of darunavir in human intestinal Caco-2 and ABCB1 gene-transfected renal LLC-PK1 cell lines. Biol Pharm Bull. 2009 Sep;32(9):1588-93.
|
| 19 |
Drug interactions with new and investigational antiretrovirals. Clin Pharmacokinet. 2009;48(4):211-41.
|
|
|
|
|
|
|