Details of the Drug Combinations
General Information of This Drug (ID: DMKINJO)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Alcomicin; Apogen; Bristagen; Cidomycin; GENTAMYCIN; Garamycin; Garasol; Gentacidin; Gentacycol; Gentafair; Gentak; Gentamar; Gentamicina; Gentamicine; Gentamicins; Gentamicinum; Gentamycinum; Gentavet; Gentocin; Jenamicin; Refobacin; Uromycine; Garamycin Otic Solution; Genoptic Liquifilm; Gentamcin Sulfate; Gentamicin sulphate sterile; Refobacin TM; Gentamicin C1; G-Mycin; G-Myticin; Garamycin (TN); Gentamicin (BAN); Gentamicin (TN); Gentamicina [INN-Spanish]; Gentamicine [INN-French]; Gentamicinum [INN-Latin];Gentamycin-creme; Gentamycin-creme [German]; Ocu-Mycin; Spectro-Genta; U-Gencin; Genoptic S.O.P.; O-2-amino-2,3,4,6,7-pentadeoxy-6-(methylamino)-alpha-D-ribo-heptopyranosyl-(1-4)-O-(3-deoxy-4-C-methyl-3-(methylamino)-beta-L-arabinopyranosyl-(1-6))-2-deoxy-D-streptamine; (1R,2S,3S,4R,6S)-4,6-diamino-3-[3-deoxy-4-C-methyl-3-(methylamino)-beta-L-arabinopyranosyloxy]-2-hydroxycyclohexyl 2-amino-2,3,4,6,7-pentadeoxy-6-(methylamino)-beta-L-lyxo-heptopyranoside; (1R,2S,3S,4R,6S)-4,6-diamino-3-{[3-deoxy-4-C-methyl-3-(methylamino)-beta-L-arabinopyranosyl]oxy}-2-hydroxycyclohexyl (6x)-2-amino-2,3,4,6,7-pentadeoxy-6-(methylamino)-alpha-D-erythro-heptopyranoside; (2R,3R,4R,5R)-2-[(1S,2S,3R,4S,6R)-4,6-diamino-3-[(2R,3R,6S)-3-amino-6-[1-(methylamino)ethyl]oxan-2-yl]oxy-2-hydroxycyclohexyl]oxy-5-methyl-4-(methylamino)oxane-3,5-diol; 2-[4,6-diamino-3-[3-amino-6-[1-(methylamino)ethyl]oxan-2-yl]oxy-2-hydroxycyclohexyl]oxy-5-methyl-4-(methylamino)oxane-3,5-diol; 4,6-diamino-3-{[3-deoxy-4-c-methyl-3-(methylamino)pentopyranosyl]oxy}-2-hydroxycyclohexyl 2-amino-2,3,4,6,7-pentadeoxy-6-(methylamino)heptopyranoside
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antibiotics
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
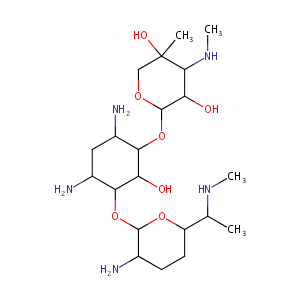 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
8 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
