Details of the Drug Combinations
General Information of This Drug (ID: DMLUA2J)
| Drug Name | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Nitropress (TN); Nitroprusside sodium; Sodium nitroprusside (USP); Tox21_110314; Tox21_110314_1; 13755-38-9; 7304AF; A-8282; C07695; NITROPRUSSIDE SODIUM DIHYDRATE; C5FeN6O.2Na.2H2O; CAS-13755-38-9; CHEBI:9179; D00614; DSSTox_CID_21126; DSSTox_GSID_41126; DSSTox_RID_79630; DTXSID7041126; MFCD00149192; NCGC00166055-03; Na2[Fe(CN)5(NO)].2H2O; Sodium nitroferricyanide dihydrate, 99+%; Sodium nitroferricyanide dihydrate, reagent ACS; Sodium nitroferricyanide(III) dihydrate; Sodium nitroprusside hydrate (JAN)
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Structure |
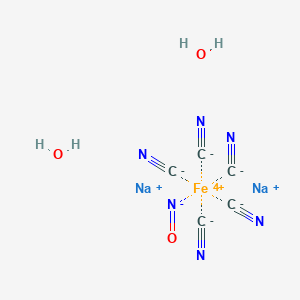 |
||||||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
