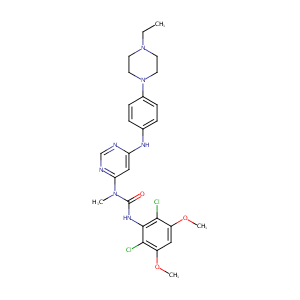
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
| DrugCom Name |
DrugCom ID |
Component Drug |
Indication |
REF |
|
BGJ398 + Panobinostat
|
DCD4QPK
|
Panobinostat
|
Diffuse intrinsic pontine glioma (Cell Line: DIPG25)
|
[3] |
|
BGJ398 + Trametinib
|
DCK2VEK
|
Trametinib
|
Embryonal rhabdomyosarcoma (Cell Line: Rh36)
|
[4] |
|
BGJ398 + Trametinib
|
DCXWIX9
|
Trametinib
|
Embryonal rhabdomyosarcoma (Cell Line: CTR)
|
[4] |
|
BGJ398 + Trametinib
|
DC0G6QD
|
Trametinib
|
Embryonal rhabdomyosarcoma (Cell Line: RD)
|
[4] |
|
BGJ398 + JQ1
|
DCJIVPA
|
JQ1
|
Embryonal rhabdomyosarcoma (Cell Line: CTR)
|
[4] |
|
BGJ398 + JQ1
|
DCUFEZC
|
JQ1
|
Embryonal rhabdomyosarcoma (Cell Line: RD)
|
[4] |
|
BGJ398 + Ceritinib
|
DC84YGE
|
Ceritinib
|
Embryonal rhabdomyosarcoma (Cell Line: Rh36)
|
[4] |
|
BGJ398 + Ceritinib
|
DCKO5SS
|
Ceritinib
|
Embryonal rhabdomyosarcoma (Cell Line: CTR)
|
[4] |
|
BGJ398 + Ceritinib
|
DCUR85F
|
Ceritinib
|
Embryonal rhabdomyosarcoma (Cell Line: RD)
|
[4] |
|
BGJ398 + GSK2126458
|
DCBMDSR
|
GSK2126458
|
Embryonal rhabdomyosarcoma (Cell Line: Rh36)
|
[4] |
|
BGJ398 + GSK2126458
|
DCXKGXA
|
GSK2126458
|
Embryonal rhabdomyosarcoma (Cell Line: CTR)
|
[4] |
|
BGJ398 + GSK2126458
|
DCH0RAL
|
GSK2126458
|
Embryonal rhabdomyosarcoma (Cell Line: RD)
|
[4] |
|
BGJ398 + BMS-754807
|
DCIKFOR
|
BMS-754807
|
Embryonal rhabdomyosarcoma (Cell Line: Rh36)
|
[4] |
|
BGJ398 + BMS-754807
|
DCF0T5U
|
BMS-754807
|
Embryonal rhabdomyosarcoma (Cell Line: CTR)
|
[4] |
|
BGJ398 + BMS-754807
|
DCCYTSA
|
BMS-754807
|
Embryonal rhabdomyosarcoma (Cell Line: RD)
|
[4] |
|
BGJ398 + MK-2206
|
DCYMFI4
|
MK-2206
|
Embryonal rhabdomyosarcoma (Cell Line: Rh36)
|
[4] |
|
BGJ398 + MK-2206
|
DCY8VM1
|
MK-2206
|
Embryonal rhabdomyosarcoma (Cell Line: CTR)
|
[4] |
|
BGJ398 + MK-2206
|
DC2QISK
|
MK-2206
|
Embryonal rhabdomyosarcoma (Cell Line: RD)
|
[4] |
|
BGJ398 + SCH772984
|
DC5HG07
|
SCH772984
|
Embryonal rhabdomyosarcoma (Cell Line: Rh36)
|
[4] |
|
BGJ398 + SCH772984
|
DCFRLD9
|
SCH772984
|
Embryonal rhabdomyosarcoma (Cell Line: CTR)
|
[4] |
|
BGJ398 + SCH772984
|
DCGIJAR
|
SCH772984
|
Embryonal rhabdomyosarcoma (Cell Line: RD)
|
[4] |
|
Everolimus + BGJ398
|
DCFH9OK
|
Everolimus
|
Embryonal rhabdomyosarcoma (Cell Line: CTR)
|
[3] |
| ------------------------------------------------------------------------------------ |
|
|
|
|