Details of the Drug Combinations
General Information of This Drug (ID: DMOWLVG)
| Drug Name | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Alinia; Colufase; Cryptaz; Daxon; Heliton; Nitazoxanid; Nitazoxanida; Nitazoxanidum; Taenitaz; Tizoxanide glucuronide; AZT + Nitazoxanide; Alinia (TN); Annita (TN); Azt+ nitazoxanide; Daxon (TN); Dexidex (TN); Kidonax (TN); Nitax (TN); Nitazox (TN); Nitazoxanida [INN-Spanish]; Nitazoxanide [USAN:INN]; Nitazoxanidum [INN-Latin]; Pacovanton (TN); Paramix (TN); Phavic-1; Zox (TN); Nitazoxanide (USAN/INN); Daxon, Dexidex, Kidonax, Pacovanton, Paramix, Nitax, Zox, Nitazoxanide
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiparasitic Agents
|
||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||
| Structure |
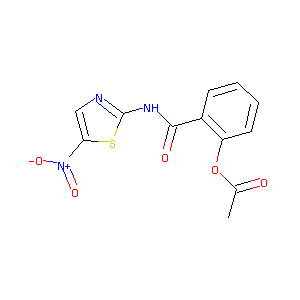 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
11 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
