Details of the Drug
General Information of Drug (ID: DMOWLVG)
| Drug Name |
Nitazoxanide
|
||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Alinia; Colufase; Cryptaz; Daxon; Heliton; Nitazoxanid; Nitazoxanida; Nitazoxanidum; Taenitaz; Tizoxanide glucuronide; AZT + Nitazoxanide; Alinia (TN); Annita (TN); Azt+ nitazoxanide; Daxon (TN); Dexidex (TN); Kidonax (TN); Nitax (TN); Nitazox (TN); Nitazoxanida [INN-Spanish]; Nitazoxanide [USAN:INN]; Nitazoxanidum [INN-Latin]; Pacovanton (TN); Paramix (TN); Phavic-1; Zox (TN); Nitazoxanide (USAN/INN); Daxon, Dexidex, Kidonax, Pacovanton, Paramix, Nitax, Zox, Nitazoxanide
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiparasitic Agents
|
||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Helminthic MicroorganismsEscherichia coliBacteria and protozoaMycobacterium tuberculosis
|
||||||||||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||
| Structure |
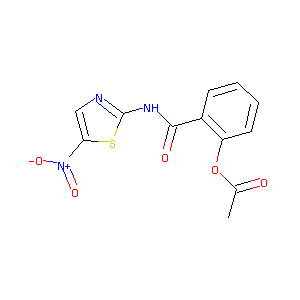 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 307.28 | |||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2 | ||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||||||||||||||||||
| ADMET Property | |||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Nitazoxanide (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Nitazoxanide FDA Label | ||||
|---|---|---|---|---|---|
| 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
| 3 | ClinicalTrials.gov (NCT04382846) Novel Regimens in COVID-19 Treatment. U.S. National Institutes of Health. | ||||
| 4 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | ||||
| 7 | The anti-hepatitis C agent nitazoxanide induces phosphorylation of eukaryotic initiation factor 2alpha via protein kinase activated by double-stranded RNA activation. Gastroenterology. 2009 Nov;137(5):1827-35. doi: 10.1053/j.gastro.2009.07.056. Epub 2009 Aug 4. | ||||
| 8 | Population-based in vitro hazard and concentration-response assessment of chemicals: the 1000 genomes high-throughput screening study. Environ Health Perspect. 2015 May;123(5):458-66. doi: 10.1289/ehp.1408775. Epub 2015 Jan 13. | ||||
| 9 | Identification of Modulators That Activate the Constitutive Androstane Receptor From the Tox21 10K Compound Library. Toxicol Sci. 2019 Jan 1;167(1):282-292. doi: 10.1093/toxsci/kfy242. | ||||
| 10 | Product Information. Alinia (nitazoxanide). Romark Laboratories L.C., Tampa, FL. | ||||
| 11 | Guo LQ, Yamazoe Y "Inhibition of cytochrome P450 by furanocoumarins in grapefruit juice and herbal medicines." Acta Pharmacol Sin 25 (2004): 129-36. [PMID: 14769198] | ||||
