Details of the Drug Combinations
General Information of This Drug (ID: DMQI43G)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Asunaprevir; 630420-16-5; BMS-650032; UNII-S9X0KRJ00S; BMS 650032; S9X0KRJ00S; Asunaprevir (BMS-650032); N-(Tert-Butoxycarbonyl)-3-Methyl-L-Valyl-(4r)-4-[(7-Chloro-4-Methoxyisoquinolin-1-Yl)oxy]-N-{(1r,2s)-1-[(Cyclopropylsulfonyl)carbamoyl]-2-Ethenylcyclopropyl}-L-Prolinamide; Asunaprevir [USAN:INN]; Sunvepra (TN); tert-butyl N-[(1S)-1-[(2S,4R)-4-[(7-chloro-4-methoxy-1-isoquinolyl)oxy]-2-[[(1R,2S)-1-(cyclopropylsulfonylcarbamoyl)-2-vinyl-cyclopropyl]carbamoyl]pyrrolidine-1-carbonyl]-2,2-dimethyl-propyl]carbamate; 2R9
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
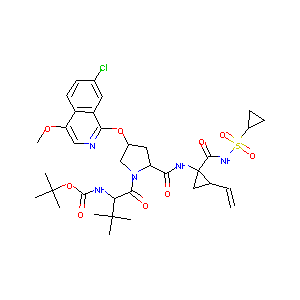 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
2 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||
References
