Details of the Drug
General Information of Drug (ID: DMQI43G)
| Drug Name |
BMS 650032
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Asunaprevir; 630420-16-5; BMS-650032; UNII-S9X0KRJ00S; BMS 650032; S9X0KRJ00S; Asunaprevir (BMS-650032); N-(Tert-Butoxycarbonyl)-3-Methyl-L-Valyl-(4r)-4-[(7-Chloro-4-Methoxyisoquinolin-1-Yl)oxy]-N-{(1r,2s)-1-[(Cyclopropylsulfonyl)carbamoyl]-2-Ethenylcyclopropyl}-L-Prolinamide; Asunaprevir [USAN:INN]; Sunvepra (TN); tert-butyl N-[(1S)-1-[(2S,4R)-4-[(7-chloro-4-methoxy-1-isoquinolyl)oxy]-2-[[(1R,2S)-1-(cyclopropylsulfonylcarbamoyl)-2-vinyl-cyclopropyl]carbamoyl]pyrrolidine-1-carbonyl]-2,2-dimethyl-propyl]carbamate; 2R9
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Hepatitis C Virus
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
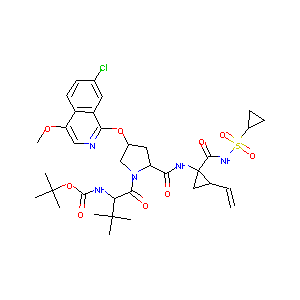 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 748.3 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 14 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 10 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
