Details of the Drug Combinations
General Information of This Drug (ID: DMQIMTK)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
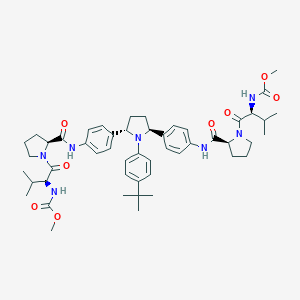
2302768XJ8; ABT 267; ABT-267; ABT-267;ABT267;ABT 267; ABT267; CHEBI:85183; CHEMBL3127326; CS-5330; DB09296; Ombitasvir; Ombitasvir (USAN); Ombitasvir [USAN:INN]; Ombitasvir pound>>ABT-267; Ombitasvir(ABT-267); SB16895; SCHEMBL8542284; UNII-2302768XJ8; ZINC150601177; dimethyl ([(2S,5S)-1-(4-tert-butylphenyl)pyrrolidine-2,5-diyl]bis{(4,1-phenylene)carbamoyl(2S)pyrrolidine-2,1-diyl[(2S)-3-methyl-1-oxobutane-1,2-diyl]})biscarbamate
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antiviral Agent
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
|
1 Approved Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
