Details of the Drug Combinations
General Information of This Drug (ID: DMQVZJD)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
ENOXIMONE; Perfan; 77671-31-9; Fenoximone; Enoximonum [Latin]; MDL-17043; Enoximona [Spanish]; MDL 17,043; UNII-C7Z4ITI7L7; MDL-17,043; RMI-17043; 4-methyl-5-(4-methylsulfanylbenzoyl)-1,3-dihydroimidazol-2-one; C7Z4ITI7L7; 4-Methyl-5-(p-(methylthio)benzoyl)-4-imidazolin-2-one; CHEMBL249856; 2H-Imidazol-2-one, 1,3-dihydro-4-methyl-5-(4-(methylthio)benzoyl)-; NCGC00015400-02; Enoximonum; Enoximona; 1,3-Dihydro-4-methyl-5-[4-methylthiobenzoyl]-2H-imidazol-2-one; DSSTox_RID_80702; DSSTox_CID_25147; DSSTox_GSID_45147; Perfane; Enoximona; E 1279; Perfan (TN); Enoximone (USAN/INN); Enoximone [USAN:BAN:INN]; 1,3-Dihydro-4-methyl-5-(4-methylthiobenzoyl)-2H-imidazol-2-one; FENOXIMONE
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Cardiotonic Agents
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
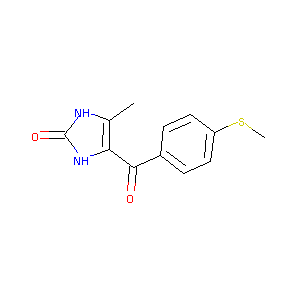 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
