Details of the Drug
General Information of Drug (ID: DMQVZJD)
| Drug Name |
Enoximone
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
ENOXIMONE; Perfan; 77671-31-9; Fenoximone; Enoximonum [Latin]; MDL-17043; Enoximona [Spanish]; MDL 17,043; UNII-C7Z4ITI7L7; MDL-17,043; RMI-17043; 4-methyl-5-(4-methylsulfanylbenzoyl)-1,3-dihydroimidazol-2-one; C7Z4ITI7L7; 4-Methyl-5-(p-(methylthio)benzoyl)-4-imidazolin-2-one; CHEMBL249856; 2H-Imidazol-2-one, 1,3-dihydro-4-methyl-5-(4-(methylthio)benzoyl)-; NCGC00015400-02; Enoximonum; Enoximona; 1,3-Dihydro-4-methyl-5-[4-methylthiobenzoyl]-2H-imidazol-2-one; DSSTox_RID_80702; DSSTox_CID_25147; DSSTox_GSID_45147; Perfane; Enoximona; E 1279; Perfan (TN); Enoximone (USAN/INN); Enoximone [USAN:BAN:INN]; 1,3-Dihydro-4-methyl-5-(4-methylthiobenzoyl)-2H-imidazol-2-one; FENOXIMONE
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Cardiotonic Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
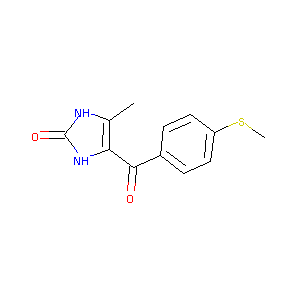 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 248.3 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Congestive heart failure | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | BD10 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
