Details of the Drug Combinations
General Information of This Drug (ID: DMSL679)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Bonoq; GTFX; Gaity; Gatiflo; Gatilox; Gatiquin; Gatispan; Tequin; Zymar; Zymaxid; Zymer; AM 1155; CG 5501;PD 135432; PD135432; AM-1155; BMS 206584-01; BMS-206584; CG-5501; Gatiflo (TN); Gatifloxacin & Gamma Interferon; Gatifloxacin (INN); Gatifloxacin (TN); Gatifloxacin [USAN:INN]; PD-135432; Tequin (TN); Tequin in dextrose 5% in plastic container; Zymar (TN); Zymer (TN); BMS-206584-01; Gatiflo,Tequin and Zymar, Gatifloxacin; AM-1155 (*Sesquihydrate*); (+-)-1-Cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic acid; 1-Cyclopropyl-1,4-dihydro-6-fluoro-8-methoxy-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic acid; 1-Cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic acid; 1-cyclopropyl-6-fluoro-8-methoxy-7-(3-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid; 1-cyclopropyl-6-fluoro-8-methoxy-7-(3-methylpiperazin-1-yl)-4-oxoquinoline-3-carboxylic acid
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiinfective Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
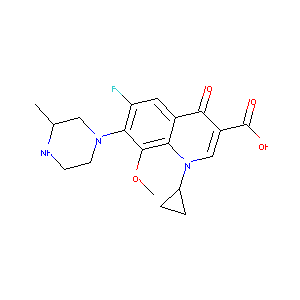 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
