Details of the Drug Combinations
General Information of This Drug (ID: DMSTH01)
| Drug Name | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
1316214-52-4; Rocilinostat (ACY-1215); 2-(Diphenylamino)-N-(7-(hydroxyamino)-7-oxoheptyl)pyrimidine-5-carboxamide; UNII-WKT909C62B; ACY-63; WKT909C62B; 2-(Diphenylamino)-N-[7-(hydroxyamino)-7-oxoheptyl]-5-pyrimidinecarboxamide; AK151416; N-[7-(hydroxyamino)-7-oxoheptyl]-2-(N-phenylanilino)pyrimidine-5-carboxamide; 2-(diphenylamino)-N-[7-(hydroxyamino)-7-oxoheptyl]pyrimidine-5-carboxamide; Ricolinostat [USAN:INN]; AH4; Ricolinostat (USAN/INN); MLS006011181; SCHEMBL574580; GTPL7010
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
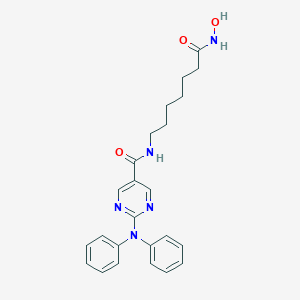 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
3 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||
References
