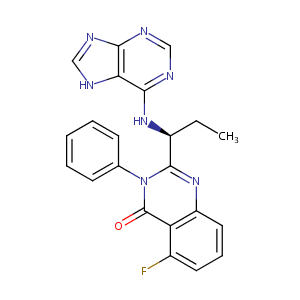
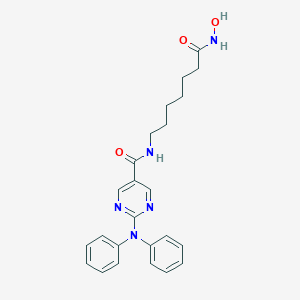
| 1 |
ClinicalTrials.gov (NCT02787369) ACY-1215 in Combination With BCR Pathway Inhibitors in Relapsed CLL
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6741).
|
| 3 |
Idelalisib FDA Label
|
| 4 |
Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800032606)
|
| 5 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7010).
|
| 6 |
2014 FDA drug approvals. Nat Rev Drug Discov. 2015 Feb;14(2):77-81.
|
| 7 |
DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018 Jan 4;46(D1):D1074-D1082. (ID: DB09054)
|
| 8 |
FDA label of Idelalisib. The 2020 official website of the U.S. Food and Drug Administration.
|
| 9 |
PI3K/AKT inhibitors aggravate death receptor-mediated hepatocyte apoptosis and liver injury. Toxicol Appl Pharmacol. 2019 Oct 15;381:114729. doi: 10.1016/j.taap.2019.114729. Epub 2019 Aug 22.
|
| 10 |
Roles of pulmonary telocytes in airway epithelia to benefit experimental acute lung injury through production of telocyte-driven mediators and exosomes. Cell Biol Toxicol. 2023 Apr;39(2):451-465. doi: 10.1007/s10565-021-09670-5. Epub 2022 Jan 3.
|
| 11 |
Induction of prolonged early G1 arrest by CDK4/CDK6 inhibition reprograms lymphoma cells for durable PI3K inhibition through PIK3IP1. Cell Cycle. 2013 Jun 15;12(12):1892-900. doi: 10.4161/cc.24928. Epub 2013 May 15.
|
| 12 |
Regulatory roles of NAT10 in airway epithelial cell function and metabolism in pathological conditions. Cell Biol Toxicol. 2023 Aug;39(4):1237-1256. doi: 10.1007/s10565-022-09743-z. Epub 2022 Jul 25.
|
| 13 |
National Cancer Institute Drug Dictionary (drug id 698408).
|
| 14 |
Preclinical activity, pharmacodynamic, and pharmacokinetic properties of a selective HDAC6 inhibitor, ACY-1215, in combination with bortezomib in multiple myeloma. Blood. 2012 Mar 15;119(11):2579-89. doi: 10.1182/blood-2011-10-387365. Epub 2012 Jan 19.
|
| 15 |
ERR contributes to HDAC6-induced chemoresistance of osteosarcoma cells. Cell Biol Toxicol. 2023 Jun;39(3):813-825. doi: 10.1007/s10565-021-09651-8. Epub 2021 Sep 15.
|
|
|
|
|
|
|