Details of the Drug Combinations
General Information of This Drug (ID: DMTAJQE)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
ABLC; Abelcet; Abelecet; Ambisome; Amphocin; Amphomoronal; Amphotec; Amphotericin; Amphotericinum; Amphotherizin; Amphozone; Fungilin; Fungisome; Fungisone; Fungizone; Halizon; SinuNase; Amfotericina B; Amophotericin B; Amphortericin B; Amphotericin B Cholesterol Dispersion; Amphotericin B Colloidal Dispersion; Amphotericin B liposomal; Amphotericin BETA; Amphotericine B; Amphotericinum B; Amphotherizin [German]; Liposomal Amphotericin B; NCI527017; NS 718; AMPH-B; Abelcet (TN); AmBisome (TN); Amfotericina B [INN-Spanish]; Ampho-Moronal; Amphocil (TN); Amphotec (TN); Amphotericin-B; Amphotericine B [INN-French]; Amphotericinum B [INN-Latin]; Fungilin (TN); Fungisome (TN); Fungizone (TN); LNS-AmB; Mysteclin-F; NKTR-024; Amphotericin B [USAN:INN:JAN]; Amphotericin B, Lipid-based; Amphotericin B, Streptomyces sp.; Amphotericin B (JP15/USP/INN)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antifungal Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
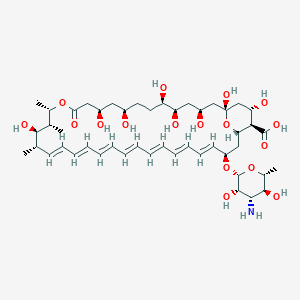 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
4 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||
References
