Details of the Drug Combinations
General Information of This Drug (ID: DMUD0IN)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms | HDP-990002; BM-21.1290; Fozivudine tidoxil; 3'-Azido-3'-deoxy-5'-O-[2-decyloxy-3-(dodecylthio)propoxy-hydroxyphosphoryl]thymidine | ||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
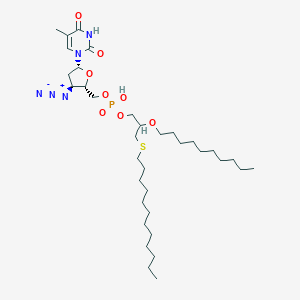 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
