Details of the Drug Combinations
General Information of This Drug (ID: DMUR64T)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
LESINURAD; 878672-00-5; RDEA594; Zurampic; RDEA 594; UNII-09ERP08I3W; RDEA-594; 2-((5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-yl)thio)acetic acid; 09ERP08I3W; AK323774; C17H14BrN3O2S; 2-[[5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-yl]thio]acetic acid; 2-[[5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-1,2,4-triazol-3-yl]sulfanyl]acetic acid; 2-(5-Bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-ylthio)acetic acid
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
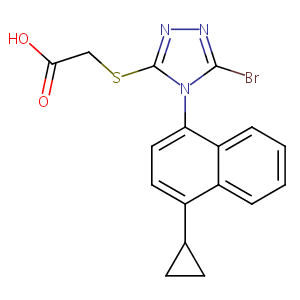 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
4 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||
References
