Details of the Drug
General Information of Drug (ID: DMUR64T)
| Drug Name |
Lesinurad
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
LESINURAD; 878672-00-5; RDEA594; Zurampic; RDEA 594; UNII-09ERP08I3W; RDEA-594; 2-((5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-yl)thio)acetic acid; 09ERP08I3W; AK323774; C17H14BrN3O2S; 2-[[5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-yl]thio]acetic acid; 2-[[5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-1,2,4-triazol-3-yl]sulfanyl]acetic acid; 2-(5-Bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-ylthio)acetic acid
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
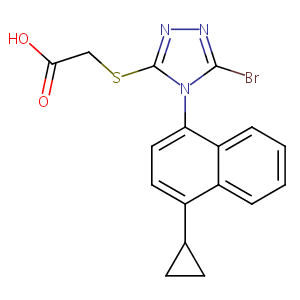 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 404.3 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Lesinurad (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7673). | ||||
|---|---|---|---|---|---|
| 2 | BDDCS predictions, self-correcting aspects of BDDCS assignments, BDDCS assignment corrections, and classification for more than 175 additional drugs | ||||
| 3 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 4 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 1031). | ||||
| 5 | FDA Label of Lesinurad. The 2020 official website of the U.S. Food and Drug Administration. | ||||
| 6 | Bell J, Seres V, Bowron P, Lewis J, Batey R "The use of serum methadone levels in patients receiving methadone maintenance." Clin Pharmacol Ther 43 (1988): 623-9. [PMID: 3378383] | ||||
| 7 | Product Information. Verzenio (abemaciclib). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 8 | Product Information. Diabinese (chlorpropamide). Pfizer US Pharmaceuticals, New York, NY. | ||||
| 9 | Product Information. Daurismo (glasdegib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 10 | Product Information. Zurampic (lesinurad). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 11 | Product Information. Aliqopa (copanlisib). Bayer Pharmaceutical Inc, West Haven, CT. | ||||
| 12 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 13 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 14 | Product Information. Pemazyre (pemigatinib). Incyte Corporation, Wilmington, DE. | ||||
| 15 | Product Information. Zepzelca (lurbinectedin). Jazz Pharmaceuticals, Palo Alto, CA. | ||||
| 16 | Product Information. Tabrecta (capmatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 17 | Product Information. Copiktra (duvelisib). Verastem, Inc., Needham, MA. | ||||
| 18 | Product Information. Calquence (acalabrutinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 19 | Product Information. Ubrelvy (ubrogepant). Allergan Inc, Irvine, CA. | ||||
| 20 | Product Information. Macrilen (macimorelin). Aeterna Zentaris, Charleston, SC. | ||||
| 21 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 22 | Product Information. Nubeqa (darolutamide). Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ. | ||||
| 23 | Product Information. Orilissa (elagolix). AbbVie US LLC, North Chicago, IL. | ||||
