Details of the Drug Combinations
General Information of This Drug (ID: DMURVE8)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Elacridar hydrochloride; 143851-98-3; Elacridar HCl; Elacridar (hydrochloride); gf 120918a; UNII-NX2BHH1A5B; NX2BHH1A5B; Elacridar hydrochloride [USAN]; GF-120918A; Elacridar hydrochloride (USAN); GF 120918; AC1Q3EOG; AC1L55DX; C34H34ClN3O5; SCHEMBL15847793; CHEMBL2074730; AOB5938; MolPort-023-332-877; BCP14056; 7066AA; AKOS016005297; CS-1113; ACN-041487; 4CA-0489; HY-50880; AC-30266; FT-0696337; W-5457; D03968; N-[4-[2-(3,4-Dihydro-6,7-dimethoxy-2(1H)-isoquinolinyl)ethyl]phenyl]-9,10-dihydro-5-methoxy-9-oxo-4-acridinecarboxamide hydro
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
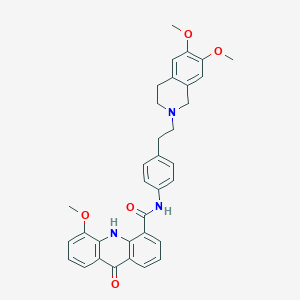 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
12 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
