Details of the Drug Combinations
General Information of This Drug (ID: DMYGEH7)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Ceftolozane; CXA-101; CXA-301; CXA-301); Cephalosporin derivatives, Astellas; Cephalosporinderivatives, Calixa Therapeutics; FR-193879; FR-264205; FR-295389; CXA-101 (inhaled), Calixa; CXA-101 (inhaled), Cubist; Cephalosporin derivative (H pylori/P aeruginosa infection), Astellas; CXA-101 (inhaled, bacterial lung infection), Cubist
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
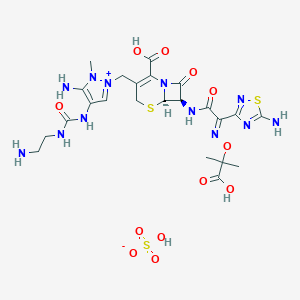 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
