Details of the Drug Combinations
General Information of This Drug (ID: DMYGHX5)
| Drug Name | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Moxidectin; ProHeart 6; UNII-NGU5H31YO9; Cydectin; NGU5H31YO9; Moxidectine [INN-French]; Moxidectinum [INN-Latin]; Moxidectina [INN-Spanish]; C37H53NO8; Moxidectina; Moxidectine; Moxidectinum; Moxidectin [USAN:INN:BAN]
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Structure |
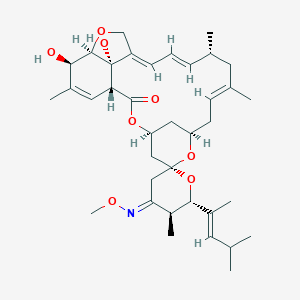 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
3 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||
References
