Details of the Drug Combinations
General Information of This Drug (ID: DMZ5RGV)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
NAPROXEN; 22204-53-1; (S)-Naproxen; Naproxene; Naprosyn; (+)-Naproxen; Equiproxen; Laraflex; Naproxenum; Naproxeno; d-Naproxen; (S)-(+)-2-(6-Methoxy-2-naphthyl)propionic acid; (S)-(+)-Naproxen; Calosen; Nycopren; Naprosyne; Bonyl; Reuxen; Naixan; Axer; (+)-(S)-Naproxen; Ec-Naprosyn; (S)-2-(6-methoxynaphthalen-2-yl)propanoic acid; Flexipen; Clinosyn; Artrixen; Anexopen; Acusprain; Novonaprox; Arthrisil; Leniartil; Danaprox; Bipronyl; Artroxen; Napren; Naposin; Napflam; Genoxen; Daprox; Atiflan; Artagen; Apronax; Naprius; Nalyxan; Lefaine; Congex
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
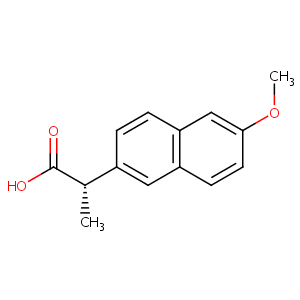 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
13 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
