Details of the Drug
General Information of Drug (ID: DMZ5RGV)
| Drug Name |
Naproxen
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
NAPROXEN; 22204-53-1; (S)-Naproxen; Naproxene; Naprosyn; (+)-Naproxen; Equiproxen; Laraflex; Naproxenum; Naproxeno; d-Naproxen; (S)-(+)-2-(6-Methoxy-2-naphthyl)propionic acid; (S)-(+)-Naproxen; Calosen; Nycopren; Naprosyne; Bonyl; Reuxen; Naixan; Axer; (+)-(S)-Naproxen; Ec-Naprosyn; (S)-2-(6-methoxynaphthalen-2-yl)propanoic acid; Flexipen; Clinosyn; Artrixen; Anexopen; Acusprain; Novonaprox; Arthrisil; Leniartil; Danaprox; Bipronyl; Artroxen; Napren; Naposin; Napflam; Genoxen; Daprox; Atiflan; Artagen; Apronax; Naprius; Nalyxan; Lefaine; Congex
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
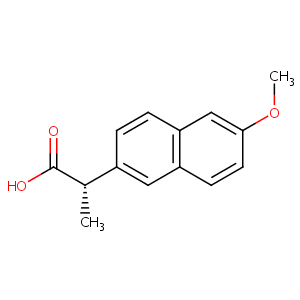 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 230.26 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Bursitis | |||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | ||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Naproxen (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Naproxen FDA Label | ||||
|---|---|---|---|---|---|
| 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
| 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5230). | ||||
| 4 | New drugs in development for the treatment of endometriosis. Expert Opin Investig Drugs. 2008 Aug;17(8):1187-202. | ||||
| 5 | Todd PA, Clissold SP: Naproxen. A reappraisal of its pharmacology, and therapeutic use in rheumatic diseases and pain states. Drugs. 1990 Jul;40(1):91-137. doi: 10.2165/00003495-199040010-00006. | ||||
| 6 | BDDCS applied to over 900 drugs | ||||
| 7 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 8 | Naprelan FDA Label | ||||
| 9 | Falany CN, Strom P, Swedmark S: Sulphation of o-desmethylnaproxen and related compounds by human cytosolic sulfotransferases. Br J Clin Pharmacol. 2005 Dec;60(6):632-40. doi: 10.1111/j.1365-2125.2005.02506.x. | ||||
| 10 | Naproxen . | ||||
| 11 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 12 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 13 | Comparative inhibitory activity of rofecoxib, meloxicam, diclofenac, ibuprofen, and naproxen on COX-2 versus COX-1 in healthy volunteers. J Clin Pharmacol. 2000 Oct;40(10):1109-20. | ||||
| 14 | Drug interactions in dentistry: the importance of knowing your CYPs. J Am Dent Assoc. 2004 Mar;135(3):298-311. | ||||
| 15 | Influence of mutations associated with Gilbert and Crigler-Najjar type II syndromes on the glucuronidation kinetics of bilirubin and other UDP-glucuronosyltransferase 1A substrates. Pharmacogenet Genomics. 2007 Dec;17(12):1017-29. | ||||
| 16 | Involvement of multiple cytochrome P450 isoforms in naproxen O-demethylation. Eur J Clin Pharmacol. 1997;52(4):293-8. | ||||
| 17 | Substrates, inducers, inhibitors and structure-activity relationships of human Cytochrome P450 2C9 and implications in drug development. Curr Med Chem. 2009;16(27):3480-675. | ||||
| 18 | S-Naproxen and desmethylnaproxen glucuronidation by human liver microsomes and recombinant human UDP-glucuronosyltransferases (UGT): role of UGT2B7 in the elimination of naproxen. Br J Clin Pharmacol. 2005 Oct;60(4):423-33. | ||||
| 19 | Biological evaluation of cobalt(II) complexes with non-steroidal anti-inflammatory drug naproxen. J Inorg Biochem. 2012 Feb;107(1):54-64. doi: 10.1016/j.jinorgbio.2011.10.014. Epub 2011 Nov 3. | ||||
| 20 | Type 5 17beta-hydroxysteroid dehydrogenase/prostaglandin F synthase (AKR1C3): role in breast cancer and inhibition by non-steroidal anti-inflammatory drug analogs. Chem Biol Interact. 2009 Mar 16;178(1-3):221-7. | ||||
| 21 | Effect of common medications on the expression of SARS-CoV-2 entry receptors in liver tissue. Arch Toxicol. 2020 Dec;94(12):4037-4041. doi: 10.1007/s00204-020-02869-1. Epub 2020 Aug 17. | ||||
| 22 | Cyclooxygenase inhibitors down regulate P-glycoprotein in human colorectal Caco-2 cell line. Pharm Res. 2008 Sep;25(9):1991-2001. doi: 10.1007/s11095-008-9596-1. Epub 2008 Jun 26. | ||||
| 23 | Early identification of clinically relevant drug interactions with the human bile salt export pump (BSEP/ABCB11). Toxicol Sci. 2013 Dec;136(2):328-43. | ||||
| 24 | Differential activation of CYP2C9 variants by dapsone. Biochem Pharmacol. 2004 May 15;67(10):1831-41. | ||||
| 25 | Effects of acidic non-steroidal anti-inflammatory drugs on human cytochrome P450 4A11 activity: Roles of carboxylic acid and a sulfur atom in potent inhibition by sulindac sulfide. Chem Biol Interact. 2023 Sep 1;382:110644. doi: 10.1016/j.cbi.2023.110644. Epub 2023 Jul 25. | ||||
| 26 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 27 | Brogden RN, Heel RC, Speight TM, Avery GS "Naproxen up to date: a review of its pharmacological properties and therapeutic efficacy and use in rheumatic diseases and pain states." Drugs 18 (1979): 241-77. [PMID: 387372] | ||||
| 28 | Abad S, Moachon L, Blanche P, Bavoux F, Sicard D, Salmon-Ceron D "Possible interaction between glicazide, fluconazole and sulfamethoxazole resulting in severe hypoglycaemia." Br J Clin Pharmacol 52 (2001): 456-7. [PMID: 11678792] | ||||
| 29 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 30 | Product Information. Sirturo (bedaquiline). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 31 | Product Information. Acular (ketorolac). Allergan Inc, Irvine, CA. | ||||
| 32 | Buchman AL, Schwartz MR "Colonic ulceration associated with the systemic use of nonsteroidal antiinflammatory medication." J Clin Gastroenterol 22 (1996): 224-6. [PMID: 8724264] | ||||
| 33 | Product Information. Factive (gemifloxacin). GeneSoft Inc, San Francisco, CA. | ||||
| 34 | Assael BM, Chiabrando C, Gagliardi L, Noseda A, Bamonte F, Salmona M "Prostaglandins and aminoglycoside nephrotoxicity." Toxicol Appl Pharmacol 78 (1985): 386-94. [PMID: 4049389] | ||||
| 35 | Product Information. Naprosyn (naproxen). Syntex Laboratories Inc, Palo Alto, CA. | ||||
| 36 | Product Information. Actonel (risedronate). Procter and Gamble Pharmaceuticals, Cincinnati, OH. | ||||
| 37 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 38 | Product Information. Tykerb (lapatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 39 | Product Information. Piqray (alpelisib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 40 | Wong GT, Lee EY, Irwin MG. Contrast induced nephropathy in vascular surgery.?Br J Anaesth. 2016;117 Suppl 2:ii63-ii73. [PMID: 27566809] | ||||
| 41 | Alderman CP, Moritz CK, Ben-Tovim DI "Abnormal platelet aggregation associated with fluoxetine therapy." Ann Pharmacother 26 (1992): 1517-9. [PMID: 1482806] | ||||
| 42 | Product Information. Yasmin (drospirenone-ethinyl estradiol) Berlex Laboratories, Richmond, CA. | ||||
| 43 | Bang CJ, Riedel B, Talstad I, Berstad A "Interaction between heparin and acetylsalicylic acid on gastric mucosal and skin bleeding in humans." Scand J Gastroenterol 27 (1992): 489-94. [PMID: 1321488] | ||||
| 44 | Product Information. Korlym (mifepristone). Corcept Therapeutics Incorporated, Menlo Park, CA. | ||||
| 45 | Product Information. Isturisa (osilodrostat). Recordati Rare Diseases Inc, Lebanon, NJ. | ||||
| 46 | Product Information. Kalydeco (ivacaftor). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 47 | Product Information. Prevymis (letermovir). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 48 | Product Information. Xarelto (rivaroxaban). Bayer Inc, Toronto, IA. | ||||
| 49 | Canadian Pharmacists Association. | ||||
| 50 | Muller FO, Schall R, Devaal AC, Groenewoud G, Hundt HKL, Middle MV "Influence of meloxicam on furosemide pharmacokinetics and pharmacodynamics in healthy volunteers." Eur J Clin Pharmacol 48 (1995): 247-51. [PMID: 7589049] | ||||
| 51 | Product Information. Priftin (rifapentine). Hoechst Marion-Roussel Inc, Kansas City, MO. | ||||
| 52 | Product Information. Adcetris (brentuximab vedotin). Seattle Genetics Inc, Bothell, WA. | ||||
| 53 | Product Information. Intelence (etravirine). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 54 | Product Information. Kynamro (mipomersen). Genzyme Corporation, Cambridge, MA. | ||||
| 55 | EMEA "EMEA public statement on leflunomide (ARAVA) - severe and serious hepatic reactions.". | ||||
| 56 | Product Information. Juxtapid (lomitapide). Aegerion Pharmaceuticals Inc, Cambridge, MA. | ||||
| 57 | Product Information. Givlaari (givosiran). Alnylam Pharmaceuticals, Cambridge, MA. | ||||
| 58 | Novis BH, Korzets Z, Chen P, Bernheim J "Nephrotic syndrome after treatment with 5-aminosalicylic acid." Br Med J (Clin Res Ed) 296 (1988): 1442. [PMID: 3132281] | ||||
| 59 | Product Information. Tabrecta (capmatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 60 | Caruso V, Iacoviello L, Di Castelnuovo A, et.al "Thrombotic complications in childhood acute lymphoblastic leukemia: a meta-analysis of 17 prospective studies comprising 1752 pediatric patients." Blood 108 (2006): 2216-22. [PMID: 16804111] | ||||
| 61 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 62 | Product Information. Calquence (acalabrutinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 63 | Agencia Espaola de Medicamentos y Productos Sanitarios Healthcare "Centro de informacion online de medicamentos de la AEMPS - CIMA.". | ||||
| 64 | Product Information. Iclusig (ponatinib). Ariad Pharmaceuticals Inc, Cambridge, MA. | ||||
| 65 | Product Information. Exjade (deferasirox). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 66 | Product Information. Sprycel (dasatinib). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 67 | Product Information. Brukinsa (zanubrutinib). BeiGene USA, Inc, San Mateo, CA. | ||||
| 68 | Product Information. Zontivity (vorapaxar). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 69 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 70 | Concomitant use of ibuprofen and aspirin. J Pain Palliat Care Pharmacother 21 (2007): 73-4. [PMID: 17844731] | ||||
| 71 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 72 | Benoist G, van Oort I, et al "Drug-drug interaction potential in men treated with enzalutamide: Mind the gap." Br J Clin Pharmacol 0 (2017): epub. [PMID: 28881501] | ||||
| 73 | Product Information. Flolan (epoprostenol). Glaxo Wellcome, Research Triangle Park, NC. | ||||
| 74 | Product Information. Prograf (tacrolimus). Fujisawa, Deerfield, IL. | ||||
| 75 | Product Information. Cometriq (cabozantinib). Exelixis Inc, S San Francisco, CA. | ||||
| 76 | Product Information. Bevyxxa (betrixaban). Portola Pharmaceuticals, South San Francisco, CA. | ||||
