| Synonyms |
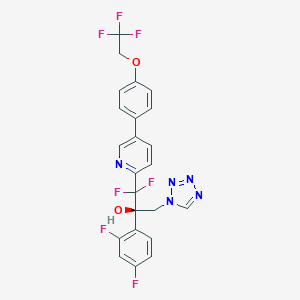
Otesaconazole; 1340593-59-0; VT-1161; UNII-VHH774W97N; VHH774W97N; CHEMBL3311228; (R)-2-(2,4-Difluorophenyl)-1,1-Difluoro-3-(1h-Tetrazol-1-Yl)-1-(5-(4-(2,2,2-Trifluoroethoxy)phenyl)pyridin-2-Yl)propan-2-Ol; Oteseconazole [USAN]; Oteseconazole (USAN/INN); SCHEMBL17113021; BDBM50046187; WHO 10138; DB13055; SB17420; HY-17643; CS-0016914; D11785; Q27291837; (2R)-2-(2,4-difluorophenyl)-1,1-difluoro-3-(1H-tetrazol-1-yl)-1-{5-[4-(2,2,2-trifluoroethoxy)phenyl]pyridin-2-yl}propan-2-ol; (2R)-2-(2,4-difluorophenyl)-1,1-difluoro-3-(tetrazol-1-yl)-1-[5-[4-(2,2,2-trifluoroethoxy)phenyl]pyridin-2-yl]propan-2-ol; 2-Pyridineethanol, alpha-(2,4-difluorophenyl)-beta,beta-difluoro-alpha-(1H-tetrazol-1-ylmethyl)-5-(4-(2,2,2-trifluoroethoxy)phenyl)-, (alphaR)-
|