Details of the Drug
General Information of Drug (ID: DM7R145)
| Drug Name |
Oteseconazole
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Otesaconazole; 1340593-59-0; VT-1161; UNII-VHH774W97N; VHH774W97N; CHEMBL3311228; (R)-2-(2,4-Difluorophenyl)-1,1-Difluoro-3-(1h-Tetrazol-1-Yl)-1-(5-(4-(2,2,2-Trifluoroethoxy)phenyl)pyridin-2-Yl)propan-2-Ol; Oteseconazole [USAN]; Oteseconazole (USAN/INN); SCHEMBL17113021; BDBM50046187; WHO 10138; DB13055; SB17420; HY-17643; CS-0016914; D11785; Q27291837; (2R)-2-(2,4-difluorophenyl)-1,1-difluoro-3-(1H-tetrazol-1-yl)-1-{5-[4-(2,2,2-trifluoroethoxy)phenyl]pyridin-2-yl}propan-2-ol; (2R)-2-(2,4-difluorophenyl)-1,1-difluoro-3-(tetrazol-1-yl)-1-[5-[4-(2,2,2-trifluoroethoxy)phenyl]pyridin-2-yl]propan-2-ol; 2-Pyridineethanol, alpha-(2,4-difluorophenyl)-beta,beta-difluoro-alpha-(1H-tetrazol-1-ylmethyl)-5-(4-(2,2,2-trifluoroethoxy)phenyl)-, (alphaR)-
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Affected Organisms |
Candida albicans and other yeasts
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
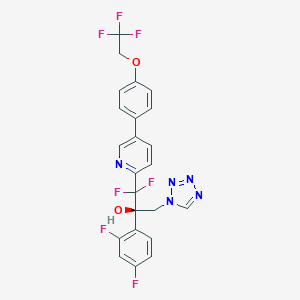 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 527.4 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.2 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 13 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
