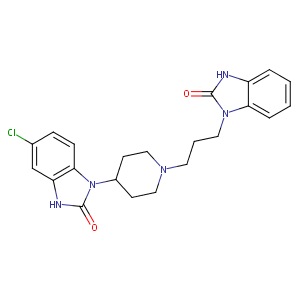
| Synonyms |
Domperidon; Domperidona; Domperidonum; Motilium; Nauzelin; KW 5338; NCI299589; Costi (TN); D-122; Domperidona [INN-Spanish]; Domperidonum [INN-Latin]; HS-0067; KW-5338; Motilium (TN); Motillium (TN); Motinorm (TN); R 33,812; R-33812; R-33,812; Domperidone (JAN/USAN/INN); Domperidone [USAN:BAN:INN:JAN]; 4-(5-Chloro-2-oxo-1-benzimidazolinyl)-1-[3-(2-oxobenzimidazolinyl)propyl]piperidine; 5-Chloro-1-(1-(3-(2-oxo-1-benzimidazolinyl)propyl)-4-piperidyl)-2-benzimidazolinone; 5-Chloro-1-[1-[3-(2,3-dihydro-2-oxo-1H-benzimidazol-1-yl)propyl]-4-piperidinyl]-1,3-dihydro-2H-benzimidazol-2-one; 5-Chloro-1-[1-[3-(2-oxo-1-benzimidazolinyl)propyl]-4-piperidyl]-2-benzimidazolinone; 5-chloro-1-(1-(3-(2-oxo-2,3-dihydro-1H-benzimidazol-1-yl)propyl)-4-piperidinyl)-1,3-dihydro-2H-benzimidazol-2-one; 5-chloro-1-{1-[3-(2-oxo-2,3-dihydro-1H-benzimidazol-1-yl)propyl]piperidin-4-yl}-1,3-dihydro-2H-benzimidazol-2-one; 6-chloro-3-[1-[3-(2-oxo-3H-benzimidazol-1-yl)propyl]piperidin-4-yl]-1H-benzimidazol-2-one
|