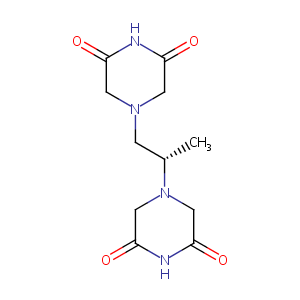
| Synonyms |
24584-09-6; Zinecard; (S)-4,4'-(Propane-1,2-diyl)bis(piperazine-2,6-dione); Cardioxane; ICRF-187; Dexrazoxano; Dexrazoxanum; Dextrorazoxane; Dexrazoxanum [INN-Latin]; Dexrazoxano [INN-Spanish]; Desrazoxane; Eucardion; ADR 529; ICRF 187; (+)-(S)-4,4'-Propylenedi-2,6-piperazinedione; Dexrazone; ADR-529; (+)-1,2-Bis(3,5-dioxo-1-piperazinyl)propane; HSDB 7319; UNII-048L81261F; NSC169780; dyzoxane; BRN 5759131; CHEBI:50223; 4-[(2S)-2-(3,5-dioxopiperazin-1-yl)propyl]piperazine-2,6-dione; NSC 169780; AK-72797; Razoxanum; Cardioxane; Dyzoxane; Savene; TopoTect; Totect; Dexrazoxane HCl; Dexrazoxane hydrochloride; ICRF 187 hydrochloride; Cardioxane (TN); Dexrazoxane (TN); Totect (TN); Zinecard (TN); Dexrazoxane (USAN/INN); Dexrazoxane [USAN:BAN:INN]; Soluble ICRF (L-isosomer); Razoxane, (S)-Isomer; Totect, ICRF-187, Zinecard, Cardioxane, Dexrazoxane Hydrochloride;(+)-(S)-4,4'-Propylenedi-2,6-piperazinedione; (+)-1,2-Bis(3,5-dioxopiperazin-1-yl)propane; (S)-(+)-1,2-Bis(3,5-dioxopiperazin-1-yl)propane; 2,6-Piperazinedione, 4,4'-(1-methyl-1,2-ethanediyl)bis-, (+)-(9CI); 4,4'-(2S)-propane-1,2-diyldipiperazine-2,6-dione; 4-[(2S)-2-(3,5-dioxopiperazin-1-yl)propyl]piperazine-2,6-dione hydrochloride; Icrf-187
|