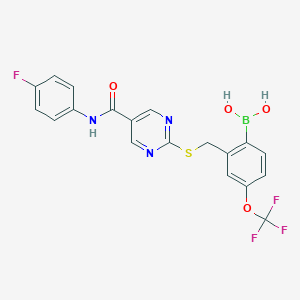
| Synonyms |
1648843-04-2; UNII-H5212R2DPM; H5212R2DPM; SX682; (2-(((5-((4-Fluorophenyl)carbamoyl)pyrimidin-2-yl)thio)methyl)-4-(trifluoromethoxy)phenyl)boronic acid; CHEMBL4297480; GTPL10165; BCP32154; EX-A4295; MFCD28502254; s8947; SX 682; SX682; SB17394; HY-119339; CS-0067128; [2-[[5-[(4-fluorophenyl)carbamoyl]pyrimidin-2-yl]sulfanylmethyl]-4-(trifluoromethoxy)phenyl]boronic acid; 2-(2-Dihydroxyboryl-5-trifluoromethoxybenzylsulfanyl)pyrimidine-5-carboxylic acid (4-fluorophenyl)amide; B-(2-(((5-(((4-Fluorophenyl)amino)carbonyl)-2-pyrimidinyl)thio)methyl)-4-(trifluoromethoxy)phenyl)boronic acid; Boronic acid, B-(2-(((5-(((4-fluorophenyl)amino)carbonyl)-2-pyrimidinyl)thio)methyl)-4-(trifluoromethoxy)phenyl)-
|