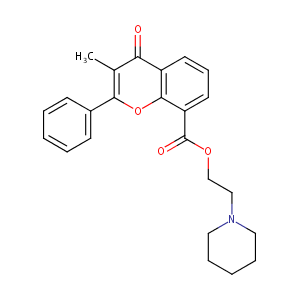
| Synonyms |
Bladuril; Flavossato; Flavoxato; Flavoxatum; Urispas; Flavossato [DCIT]; Flavoxate HCI; Flavoxate HCL; Spasuret hydrochloride; AK 123; Bladderon (TN); Bladuril (TN); DW-61; Flavoxate (INN); Flavoxate [INN:BAN]; Flavoxato [INN-Spanish]; Flavoxatum [INN-Latin]; Urispas (TN); Uritac (TN); Zopyran-8-carboxylate; Beta-piperidinoethyl 3-methylflavone-8-carboxylate; Piperidinoethyl-3-methylflavone-8-carboxylate; 1-Piperidinoethanol, 3-methyl-4-oxo-2-phenyl-4H-1-benzopyran-8-carboxylate; 2-(1-Piperidinyl)ethyl 3-methyl-4-oxo-2-phenyl-4H-chromene-8-carboxylate; 2-(piperidin-1-yl)ethyl 3-methyl-4-oxo-2-phenyl-4H-chromene-8-carboxylate; 2-Piperidinoethyl 3-methyl-4-oxo-2-phenyl-4H-1-benzopyran-8-carboxylat; 2-Piperidinoethyl 3-methyl-4-oxo-2-phenyl-4H-1-benzopyran-8-carboxylate; 2-Piperidinoethyl-3-methyl-4-oxo-2-phenyl-4H-1-ben; 2-piperidin-1-ylethyl 3-methyl-4-oxo-2-phenyl-4H-chromene-8-carboxylate; 2-piperidin-1-ylethyl 3-methyl-4-oxo-2-phenylchromene-8-carboxylate; 2-piperidinoethyl 3-methylflavone-8-carboxylate; 4H-1-Benzopyran-8-carboxylic acid, 3-methyl-4-oxo-2-phenyl-, 2-(1-piperidinyl)ethyl ester
|