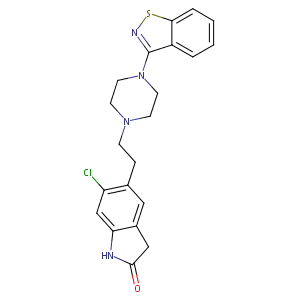
| Synonyms |
146939-27-7; Geodon; Zeldox; Ziprasidone hydrochloride; Ziprasidone [INN:BAN]; UNII-6UKA5VEJ6X; CP 88059; Ziprasidone mesylate trihydrate; 6UKA5VEJ6X; CHEMBL708; C21H21ClN4OS; CHEBI:10119; CP-88,059-1; ziprazidone; DSSTox_CID_3753; DSSTox_RID_77186; 5-[2-[4-(1,2-benzothiazol-3-yl)piperazin-1-yl]ethyl]-6-chloro-1,3-dihydroindol-2-one; DSSTox_GSID_23753; ziprasidonum; ziprasidona; 5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)ethyl)-6-chloroindolin-2-one; Geodon; Zipradon; Ziprasidona; Ziprasidonum; Ziprazidone; CP 88059-01; Geodon (TN); Zeldox (TN); Zipradon (TN); Ziprasidone (INN); CP-88,059; CP-88059-1; CP-88,059-01; 5-{2-[4-(1,2-benzisothiazol-3-yl)piperazin-1-yl]ethyl}-6-chloro-1,3-dihydro-2H-indol-2-one; 5-{2-[4-(1,2-benzothiazol-3-yl)piperazin-1-yl]ethyl}-6-chloro-1,3-dihydro-2H-indol-2-one; 6-chloro-5-[2-[4-(7-thia-8-azabicyclo[430]nona-1,3,5,8-tetraen-9-yl)piperazin-1-yl]ethyl]-1,3-dihydroindol-2-one; TC-5280
|