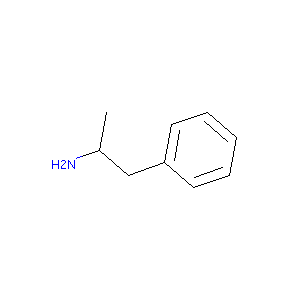
| Synonyms |
D-Amphetamine; dextroamphetamine; Dexamphetamine; Dexamfetamine; (S)-Amphetamine; Dexadrine; (+)-(S)-Amphetamine; Dexedrine; (2S)-1-phenylpropan-2-amine; (S)-(+)-Amphetamine; Dextrostat; Desamfetamina; (S)-1-Phenyl-2-propylamine; Dexidrine; Sympamin; Dephadren; Amsustain; (+)-Phenaminum; (+)-alpha-Methylphenethylamine; (S)-1-Phenyl-2-aminopropane; (+)-alpha-Methylphenylethylamine; D-(S)-Amphetamine; (S)-(+)-beta-Phenylisopropylamine; 51-64-9; (S)-alpha-Phenylethylamine; (2S)-(+)-Amphetamine; Benzedrine; Dexacaps; Dexamfetamina; Dexamfetaminum; Dexamphetaminum; Dexanfetamina; Isoamycin; Propisamine; Psychedrine; Raphetamine; Rhinalator; Simpatedrin; Sympamine; Sympatedrine; Weckamine; Desamfetamina [DCIT]; Dexedrine Spansule; Dextroamphetamine [USAN]; Dextroamphetamine resin complex; D-AM; Dexamfetamina [INN-Spanish]; Dexamfetamine (INN); Dexamfetaminum [INN-Latin]; Dexamphetaminum [INN-Latin]; Dexanfetamina [INN-Spanish]; Dexedrine (TN); Dextro-Amphetamine; Dextro-Amphetamine Sulfate; Dextroamphetamine (USAN); Dextrostat (TN); Dl-Amphetamine; Dl-Benzedrine; Fenylo-izopropylaminyl; Beta-phenyl-isopropylamine; D-alpha-methylphenethylamine; Alpha-Methylphenethylamine, d-form; S(+)-Amphetamine; D-(+)-Amphetamine; D-1-Phenyl-2-aminopropan; D-1-Phenyl-2-aminopropan [German]; D-1-Phenyl-2-aminopropane; D-2-Amino-1-phenylpropane; Dl-1-Phenyl-2-aminopropane; Benzeneethanamine, alpha-methyl-, (aS)-(9CI); Phenethylamine, alpha-methyl-, (+)-(8CI); (+/-)-Benzedrine; (+/-)-Desoxynorephedrine; (+/-)-beta-Phenylisopropylamine; (S)-1-Phenyl-2-propanamine; (S)-alpha-Methylphenethylamine; (S)-alpha-methylbenzeneethanamine; (alphaS)-alpha-methylbenzeneethanamine; dextroamphetamine sulfate (oral liquid, ADHD), Auriga
|