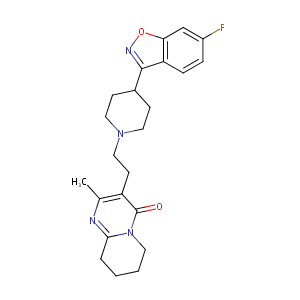
| Synonyms |
Belivon; Risperdal; Risperdone; Risperidal; Risperidona; Risperidonum; Risperin; Rispolept; Rispolin; Sequinan; Risperdal Consta; Risperidona [Spanish]; Risperidonum [Latin]; R 62 766; R 64766; R64766; Consta, Risperdal; KS-1106; R 64,766; R-118; R-64766; R64,766; Risperdal (TN); Risperdal M-Tab; Risperidal M-Tab; Risperidone (RIS); Risperidone, placebo; R 64 766, Risperdal, Risperidone; R-64,766; R-64-766; Risperidone [USAN:BAN:INN]; Risperidone (JAN/USAN/INN); 3-(2-(4-(6-Fluoro-1,2-benzisoxazol-3-yl)piperidino)ethyl)-6,7,8,9-tetrahydro-2-methyl-4H-pyrido(1,2-a)pyrimidin-4-one; 3-[2-[-4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one; 3-[2-[4-(6-Fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido [1,2-a] pyrimidin-4-one; 3-[2-[4-(6-Fluoro-1,2-benzisoxazol-3-yl)piperi-dino]ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]-pyrimidin-4-one; 3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one; 3-[2-[4-(6-fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl]-2-methyl-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-4-one; 3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperidin-1-yl]-ethyl}-2-methyl-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-4-one; 3-{2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)piperidin-1-yl]ethyl}-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one; 3-{2-[4-(6-fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl}-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one
|