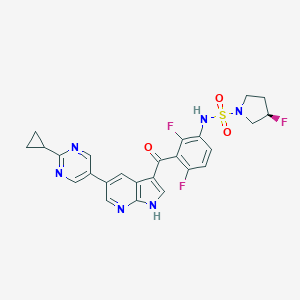
Details of the Drug Reposition
Information on Drug Reposition of This Drug
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
1 Phase 2 Indication(s)
|
|
|||||||||||||||||||||||||
|
1 Phase 1/2 Indication(s)
|
|
|||||||||||||||||||||||||
References