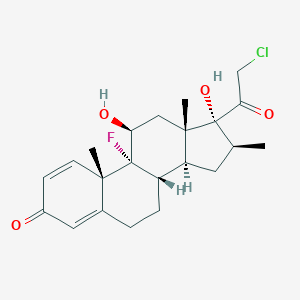
| Synonyms |
Clobetasolum; Clofenazon; Clobecort Amex; Butavate (TN); Clobecort Amex (TN); Clobetasol (INN); Clobetasol [INN:BAN]; Clobetasolum [INN-Latin]; Clobex (TN); Dermatovate (TN); Dermovate (TN); Movate (TN); Olux (TN); Temovate (TN); Tenovate (TN); (11beta,16beta)-21-chloro-9-fluoro-11,17-dihydroxy-16-methylpregna-1,4-diene-3,20-dione; (8S,9R,10S,11S,13S,14S,16S,17R)-17-(2-chloroacetyl)-9-fluoro-11,17-dihydroxy-10,13,16-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one; (9R,17R)-17-(2-chloroacetyl)-9-fluoro-11,17-dihydroxy-10,13,16-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one; 17-(2-chloroacetyl)-9-fluoro-11,17-dihydroxy-10,13,16-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one; 21-Chloro-9-fluoro-11beta,17-dihydroxy-16beta-methylpregna-1,4-diene-3,20-dione; 21-chloro-9-fluoro-11,17-dihydroxy-16-methylpregna-1,4-diene-3,20-dione
|