Details of the Drug
General Information of Drug (ID: DM086S6)
| Drug Name |
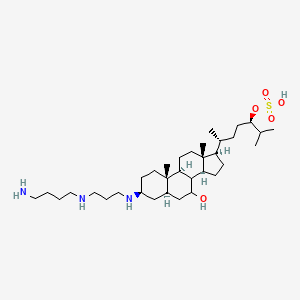
ENT-01
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
ENT-01; ENT01; GLXC-26740; (3R,6R)-6-((3S,5R,7R,9S,10S,13R,14S,17R)-3-((3-((4-Aminobutyl)amino)propyl)amino)-7-hydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)-2-methylheptan-3-yl hydrogen sulfate
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||
| Structure |
 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
References
