| Synonyms |
136701-68-3; RP-68303; CHEMBL274728; 1-(2-(4-((5-Fluoro-1H-indol-3-yl)methyl)-1-piperidinyl)ethyl)-5,6-dihydro-1H,4H-1,2,5-thiadiazolo(4,3,2-ij)quinoline 2,2-dioxide; 1-(Fim-PE)-dtqd; 1H,4H-[1,2,5]Thiadiazolo[4,3,2-ij]quinoline,1-[2-[4-[(5-fluoro-1H-indol-3-yl)methyl]-1-piperidinyl]ethyl]-5,6-dihydro-,2,2-dioxide; ACMC-20mw9l; AC1L4DLH; SCHEMBL9635292; CTK4C0419; DTXSID70159892; BDBM50047098; 1-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1-yl]-ethyl}-4,5-dihydro-1H,3H-2-thia-1,2a-diaza-acenaphthylene 2,2-dioxide
|
| Chemical Identifiers |
- Formula
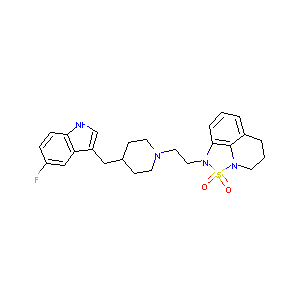
- C25H29FN4O2S
- IUPAC Name
3-[2-[4-[(5-fluoro-1H-indol-3-yl)methyl]piperidin-1-yl]ethyl]-2lambda6-thia-1,3-diazatricyclo[6.3.1.04,12]dodeca-4,6,8(12)-triene 2,2-dioxide - Canonical SMILES
-
C1CC2=C3C(=CC=C2)N(S(=O)(=O)N3C1)CCN4CCC(CC4)CC5=CNC6=C5C=C(C=C6)F
- InChI
-
InChI=1S/C25H29FN4O2S/c26-21-6-7-23-22(16-21)20(17-27-23)15-18-8-11-28(12-9-18)13-14-29-24-5-1-3-19-4-2-10-30(25(19)24)33(29,31)32/h1,3,5-7,16-18,27H,2,4,8-15H2
- InChIKey
-
NZUSTSFFNOEAHE-UHFFFAOYSA-N
|