Details of the Drug
General Information of Drug (ID: DM0KUMX)
| Drug Name |
2-(4-methoxyphenyl)-4,5-dihydro-1H-imidazole
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
2-(4-Methoxyphenyl)-4,5-dihydro-1H-imidazole; 6302-84-7; 4,5-Dihydro-2-(4-methoxyphenyl)-1H-Imidazole; 2-Imidazoline, 2-(p-methoxyphenyl)-; 1H-Imidazole, 4,5-dihydro-2-(4-methoxyphenyl)-; CHEMBL13633; NSC41538; AC1L5ZGN; 2-(p-Anisyl)-1-imidazoline; SCHEMBL9575256; AC1Q580G; CTK5B7166; DTXSID40285359; LPRQSQCAHSRGRZ-UHFFFAOYSA-N; ZINC1672770; BDBM50240364; NSC-41538; AKOS022491172; MCULE-4518622050; 1-(2-imidazolin-2-yl)-4-methoxybenzene; 2-(P-METHOXY-PHENYL)-IMIDAZOLINE; DB-073247; KB-222983; ST50998435; FT-0712566
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
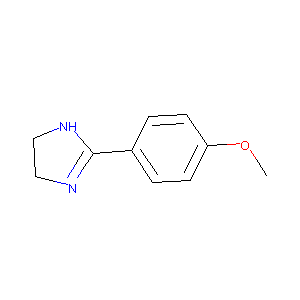 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 176.21 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
