Details of the Drug
General Information of Drug (ID: DM1AEH4)
| Drug Name |
Avibactam
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Avibactam; Avibactam (free acid); Avibactam [USAN:INN]; Avibactam free acid; AVE-1330A free acid; NXL 104; NXL104; Q15410251; SCHEMBL1666807; ZINC9302239; (2S,5R)-7-oxo-6-(sulfooxy)-1,6-diazabicyclo[3.2.1]octane-2-carboxamide; 1,6-Diazabicyclo(3.2.1)octane-2-carboxamide, 7-oxo-6-(sulfooxy)-, (1R,2S,5R)-rel-; 1192500-31-4; 396731-14-9; BDBM50339145; CHEBI:85984; CHEMBL1689063; CS-0593; DB09060; HY-14879; UNII-06MFO7817I component NDCUAPJVLWFHHB-UHNVWZDZSA-N; UNII-7352665165
|
|||||
| Affected Organisms |
Escherichia coliKlebsiellaProteus mirabilisEnterobacterPseudomonas aeruginosaCitrobacterProteus vulgarisProvidencia stuartii
|
|||||
| Structure |
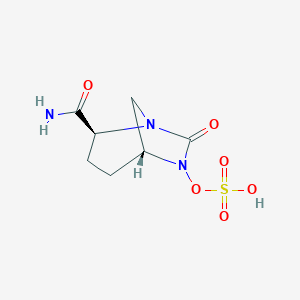 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 265.25 | ||||
| Logarithm of the Partition Coefficient (xlogp) | -1.8 | |||||
| Rotatable Bond Count (rotbonds) | 3 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | |||||
| ADMET Property |
|
|||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
