Details of the Drug
General Information of Drug (ID: DM1DVXB)
| Drug Name |
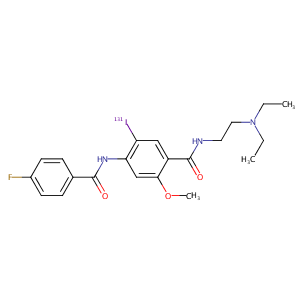
Ioflubenzamide (131I)
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Solazed; Ioflubenzamide I-131; MIP-1104; MIP-1143; MIP-1144; MIP-1145; ZK-BA; Iodine-123-Solazed; Iodine-131-Solazed; MIP-1145-[123I]; MIP-1145-[131I]; Radiolabeled benzamides (melanoma), Bayer; Radiolabeled benzamides (melanoma), Molecular Insight; Iodine-123-ZK-BA; Iodine-131-ZK-BA; Melanin-targeting radiodiagnostic (melanoma), Bayer/Molecular Insight; Melanin-targeting radiotherapeutic (melanoma), Bayer/Molecular Insight; 123I-MIP-1145; 123I-Solazed; 123I-ZK-BA
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Structure |
 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
