Details of the Drug
General Information of Drug (ID: DM1MFTZ)
| Drug Name |
BETA-CCM
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
beta-CCM; Methyl beta-carboline-3-carboxylate; beta-Carboline-3-carboxylic acid methyl ester; 69954-48-9; 3-Carbomethoxy-beta-carboline; UNII-I2A008F6YL; methyl 9H-pyrido[3,4-b]indole-3-carboxylate; CHEMBL453066; CHEMBL268191; I2A008F6YL; Methyl 9H-Pyrido(3,4-b)indole-3-carboxylate; methyl 9H-beta-carboline-3-carboxylate; 9H-beta-Carboline-3-carboxylic acid methyl ester; 9H-Pyrido(3,4-b)indole-3-carboxylic acid, methyl ester; C13H10N2O2; Beta CCM; Lopac-E-002; Biomol-NT_000273; AC1L32LA; Lopac0_000523; SCHEMBL1066725
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
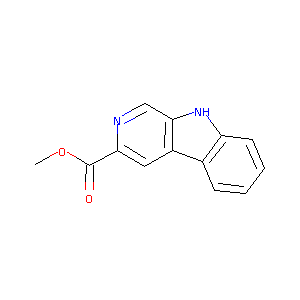 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 226.23 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
References
