Details of the Drug
General Information of Drug (ID: DM27ZJG)
| Drug Name |
AKST4290
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Lazucirnon; ALK4290; ALK-4290; AKST4290; 1251528-23-0; Lazucirnon [USAN]; ALK429; AKST-4290; R0T9LLR4TN; BI144807; ALK-429; Lazucirnon (USAN); 2-[[(2R)-1-[1-[(4-chloro-3-methylphenyl)methyl]piperidin-4-yl]-5-oxopyrrolidine-2-carbonyl]amino]-N,N,6-trimethylpyridine-4-carboxamide; 2-((((2R)-1-(1-((4-Chloro-3-methylphenyl)methyl)-4-piperidinyl)-5-oxo-2-pyrrolidinyl)carbonyl)amino)-N,N,6-trimethyl-4-pyridinecarboxamide; 4-Pyridinecarboxamide, 2-((((2R)-1-(1-((4-chloro-3-methylphenyl)methyl)-4-piperidinyl)-5-oxo-2-pyrrolidinyl)carbonyl)amino)-N,N,6-trimethyl-; LAZUCIRNON [INN]; UNII-R0T9LLR4TN; SCHEMBL1668125; CHEMBL3670800; GTPL10653; BDBM123072; GLXC-26875; WHO 11481; AKOS040755379; DB15269; MS-29506; Example 11 [WO2012045803A1]; HY-136788; CS-0133685; D11741; US8742115, 11; 2-{(2R)-1-[1-(4-Chloro-3-methylbenzyl)piperidin-4-yl]-5-oxopyrrolidin-2-carboxamido}-N,N,6-trimethylpyridine-4-carboxamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||
| Structure |
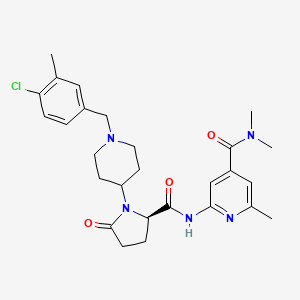 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Parkinson disease | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 8A00.0 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
