Details of the Drug
General Information of Drug (ID: DM2CEWT)
| Drug Name |
Tyr-D-Phe-Gly-D-Trp-NMeNle-Asp-Phe-NH2
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Tyr-D-Phe-Gly-D-Trp-NMeNle-Asp-Phe-NH2; CCK-Opioid Peptide, 10; CHEMBL218327; BDBM21140; H-Tyr-DPhe-Gly-DTrp-NMeNle-Asp-Phe-NH(2); (3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamido]-3-phenylpropanamido]acetamido}-3-(1H-indol-3-yl)-N-methylpropanamido]hexanamido]-3-{[(1S)-1-carbamoyl-2-phenylethyl]carbamoyl}propanoic acid
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
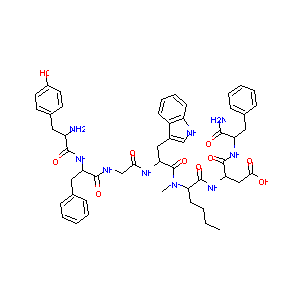 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 4 | Molecular Weight (mw) | 960.1 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 26 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 10 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 11 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
