Details of the Drug
General Information of Drug (ID: DM32GSJ)
| Drug Name |
Incruse Ellipta
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Umeclidinium bromide; 869113-09-7; Umeclidinium (bromide); GSK573719A; Incruse Ellipta; UNII-7AN603V4JV; 7AN603V4JV; GSK-573719; 1-[2-(Benzyloxy)ethyl]-4-(hydroxydiphenylmethyl)-1-quinuclidinium Bromide; 1-(2-(benzyloxy)ethyl)-4-(hydroxydiphenylmethyl)quinuclidin-1-ium bromide; CHEMBL523299; Umeclidinium brom; Umeclidinium bromide [USAN:INN]; umeclidinii bromidum; 1-Azoniabicyclo[2.2.2]octane, 4-(hydroxydiphenylmethyl)-1-[2-(phenylmethoxy)ethyl]-, bromide (1:1); 1-Azoniabicyclo(2.2.2)octane, 4-(hydroxydiphenylmethyl)-1-(2
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
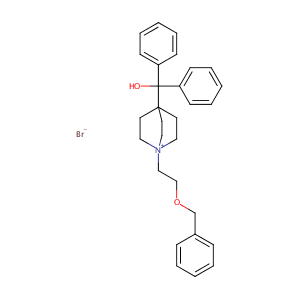 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 |
Molecular Weight | 508.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 8 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 3 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
