Details of the Drug
General Information of Drug (ID: DM3GS2X)
| Drug Name |
S-2238
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
D-Phenylalanyl-L-2-piperidinecarbonyl-N-(4-nitro phenyl)-L-argininamide; H-D-Phe-pip-arg-pna; Chromogenic substrate S-2238; D-Phe-Pip-Arg-PNA; D-F-Pip-R-PNA; H-Phe-pip-arg-pna; D-Phe-Pip-Arg-paranitroanilide; H-Phe-pip-arg-p-nitroanilide; S 2238; H-D-Phenylalanyl-pip-arg-p-nitroanilide; AC1Q5KFD; AC1L3XF1; H-D-Phenylalanyl-L-pipecolyl-arginine-nitroanilide; (2s)-n-{(2s)-5-[(diaminomethylidene)amino]-1-[(4-nitrophenyl)amino]-1-oxopentan-2-yl}-1-(d-phenylalanyl)piperidine-2-carboxamidato(3-); BDBM12678; YDMBNDUHUNWWRP-VJBWXMMDSA-N; BDBM233013; ZINC14950391
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
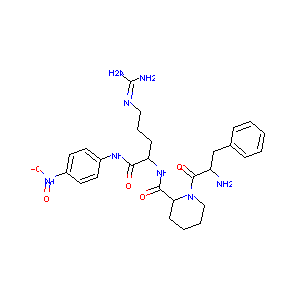 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 552.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 11 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
