Details of the Drug
General Information of Drug (ID: DM3HN3U)
| Drug Name |
Afimetoran
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Afimetoran; 2171019-55-7; Afimetoran [USAN]; LXP7MZL0VF; BMS-986256; UNII-LXP7MZL0VF; WHO 11516; BMS 986256; 2-(4-(2-(7,8-Dimethyl-[1,2,4]triazolo[1,5-a]pyridin-6-yl)-3-isopropyl-1H-indol-5-yl)piperidin-1-yl)acetamide; 1-Piperidineacetamide, 4-(2-(7,8-dimethyl(1,2,4)triazolo(1,5-a)pyridin-6-yl)-3- (1-methylethyl)-1H-indol-5-yl)-; 2-(4-(2-(7,8-dimethyl(1,2,4)triazolo(1,5-a)pyridin-6-yl)-3-(propan-2-yl)-1H-indol-5-yl)piperidin-1-yl)acetamide; AFIMETORAN [INN]; CHEMBL4650329; SCHEMBL19761011; BDBM273241; EX-A6745; US10071079, Example 15; AKOS040757385; MS-28017; HY-139567; CS-0213544; 1-PIPERIDINEACETAMIDE, 4-(2-(7,8-DIMETHYL(1,2,4)TRIAZOLO(1,5-A)PYRIDIN-6-YL)-3-(1-METHYLETHYL)-1H-INDOL-5-YL)-; 2-(4-(2-(7,8-DIMETHYL(1,2,4)TRIAZOLO(1,5-A)PYRIDIN-6-YL)-3-(PROPAN-2-YL)-1H-INDOL- 5-YL)PIPERIDIN-1-YL)ACETAMIDE; 2-(4-(2-(7,8-dimethyl-[1,2,4]triazolo[1,5-a]pyridin-6-yl)-3-isopropyl-1H-indol-5-yl) piperidin-1-yl)acetamide; 2-[4-[2-(7,8-dimethyl-[1,2,4]triazolo[1,5-a]pyridin-6-yl)-3-propan-2-yl-1H-indol-5-yl]piperidin-1-yl]acetamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||
| Structure |
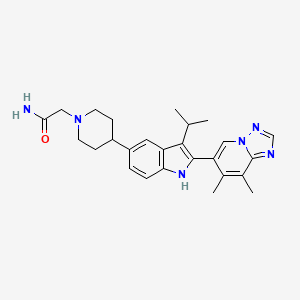 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Systemic lupus erythematosus | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 4A40.0 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
