Details of the Drug
General Information of Drug (ID: DM3J99W)
| Drug Name |
AC-1204
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
isoindoline hydrochloride; 32372-82-0; 2,3-Dihydroisoindole hydrochloride; 2,3-dihydro-1H-isoindole hydrochloride; 2,3-Dihydro-1H-isoindole HCl; Isoindoline HCl salt; 1H-Isoindole, 2,3-dihydro-, hydrochloride; Isoindoline hydrochloride, 97%; Isoindolinehydrochloride; Isoindoline, HCl; ISOINDOLINE HCL; AC1Q38WR; dihydroisoindole hydrochloride; KSC491I3F; AMBZ0192; SCHEMBL4702076; CTK3J1432; DTXSID50487241; MolPort-003-986-749; NOVIRODZMIZUPA-UHFFFAOYSA-N; BH168; CS-D1516; ACT08858; ACN-S003258; KS-000001RA
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
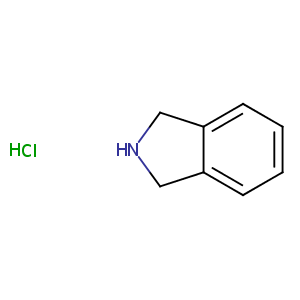 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
